#1
Multiple Sclerosis Therapy
van Oosten BW, Truyen L, Barkhof F, Polman CH
Drugs 1995 Feb;49(2):200-12
Free Univ Hospital, Dept of Neurology,
Amsterdam, The Netherlands
PMID# 7729328; UI# 95246624
Abstract
A growing amount of evidence suggests that a disturbance of Immunological function is of importance in the PathoGenesis of Multiple Sclerosis. This is reflected in the drugs used to slow progression and to treat relapses.
ImmunoSuppressive drugs such as Azathioprine, Cyclophosphamide and Cyclosporine might have some potential to slow down progression of Multiple Sclerosis, but their use is limited by potentially serious adverse effects.
Recently, it was shown that Interferon-ß-1b can diminish the exacerbation rate in Multiple Sclerosis without leading to unacceptable adverse effects. Nevertheless, symptomatic treatment remains of crucial importance in the management of Multiple Sclerosis patients.
Spasticity, Depression, Fatigue and Urinary, Paroxysmal and Sensory symptoms can all be alleviated to some extent with pharmacological interventions, although rehabilitation procedures and psychosocial consultations are no less important.
Further therapeutic approaches to Multiple Sclerosis will be directed at either the specificity of the Immune Response or the grade of activation of the Immune Response.
Magnetic Resonance Imaging techniques will play an important role in the evaluation of efficacy of new therapeutic agents.
#2
Current Therapy Of Multiple Sclerosis
Francis DA
J Clin Pharm Ther 1993 Apr;18(2):77-84
Queen Elizabeth Medical Centre, Dept of Neurology,
Birmingham, UK
PMID# 8458884; UI# 93210002
Abstract
The mainstay of treatment for Multiple Sclerosis in the U.K., and worldwide, remains CorticoSteroid therapy. High-dose pulses of IntraVenous MethylPrednisolone is currently the most favored agent for acute exacerbations or a sudden acceleration in clinical course.
Many patients who follow a more insidious decline rely on symptomatic treatments designed to ameliorate the chronic symptoms associated with their condition. Attempts to influence disease progression using a wide range of ImmuneModulating agents have not to date been of sufficient clinical benefit to justify their routine usage.
With increasing understanding of the underlying disease mechanisms future treatments are being more specifically directed toward disease prevention.
#3
Steroids, ImmunoSuppressions & Effects Of Interferon-ß-1b
In Multiple Sclerosis
Goodkin DE
West J Med 1994 Sep;161(3):292-8
Multiple Sclerosis Center,
Mount Zion Medical Center,
Univ of California, San Francisco,
School of Medicine
San Francisco, CA 94115
PMID# 7975569; UI# 95066033
Abstract
CorticoSteroids, Corticotropin, Azathioprine, Cyclophosphamide, and IFN-ß 1b have each had a substantial effect on the care of patients with Multiple Sclerosis and the design of subsequent clinical trials of experimental therapeutics for MS.
The use of MRI scanning and more sensitive clinical outcome measures will possibly enable us to complete clinical trials in a fraction of the time required for earlier trials.
The release of Betaseron, which favorably alters the attack rate in ambulatory patients with Relapsing/Remitting MS has brought a sense of renewed optimism to patients with MS, their families, and their care providers.
New promising therapies for Chronic/Progressive MS and biologic products possibly capable of enhancing the effects of IFN-ß 1b in patients with Relapsing MS are setting the stage for additional important therapeutic advances in this disease.
#4
Multiple Sclerosis Therapy With ß-1b Interferon
Initial results with 30 Multiple Sclerosis patients in northwest Switzerland
Huber S, Spycher M, Lechner-Scott J, Bellaiche Y, Steck AJ, Kappos L
Schweiz Med Wochenschr 1996 Aug 31;126(35):1475-81
Neurologische Universitatklinik,
Kantonsspital Basel
PMID# 8927950; UI# 96398403
Abstract
Recombinant Interferon-ß-1b has been registered with the Swiss health authorities since August 1995. Due to a special arrangement with health insurances it has been possible to prescribe this medication since spring 1995.
We report on our experience with the first 30 consecutively treated Multiple Sclerosis patients. Indication, adverse event profile and clinical response to treatment are described. The most common side effects were local injection site reactions (63%), influenza-like symptoms (50%) and fatigue (33%).
As compared to the prestudy period we observed a 49% reduction in the exacerbation rate. Compliance was excellent, possibly due to strict selection and extensive information about possible effects and side effects.
#5
Newer Versus Older Treatments For Relapsing/Remitting Multiple Sclerosis
Weinstock-Guttman B, Cohen JA
Drug Saf 1996 Feb;14(2):121-30
Mellen Center for Multiple Sclerosis Treatment and Research,
Cleveland Clinic Foundation,
Ohio, USA
PMID# 8852526; UI# 97005215
Abstract
Multiple Sclerosis (MS) is a chronic disease with an unpredictable clinical course and several distinct clinical patterns. Recent developments in Immunology, Molecular Biology and Genetics have improved our understanding of the PathoPhysiology of MS.
Further, advances in trial methodology, including the availability of Magnetic Resonance Imaging (MRI) as a surrogate outcome measure, have led to the identification of several new therapeutic options for Relapsing/Remitting (RR) MS.
These therapies include CorticoSteroids, recombinant Interferon-ß-1b (IFN-ß-1b), recombinant Interferon-ß-1a (IFN-ß-1a) and Copolymer-1 (Cop-1).
CorticoSteroids have been shown to accelerate the recovery from acute exacerbations, but there are still conflicting data on their effect on outcome and long term course. IFN-ß-1b, IFN-ß-1a and Cop-1 all effectively alter the natural history of RR-MS.
These 3 agents all decrease the relapse rate by approximately one-third, but differ in their adverse effect profiles and administration regimens. Further trials are required to define the optimal treatment of RR-MS.
#6
Current & Investigational Therapies To Alter The Course of Multiple Sclerosis
Miller A
South Med J 1997 Apr;90(4):367-75
Maimonides Medical Center, Division of Neurology,
Brooklyn, NY 11219, USA
PMID# 9114824; UI# 97269926
Abstract
Extensive research is under way to develop pharmacotherapeutic agents that will prevent the exacerbations and the progression of neurologic disability associated with Multiple Sclerosis (MS).
The most intensive research strategy has involved agents intended to limit DeMyelination by reducing inflammation and modifying the Immune Response. In this category are Interferon-ß-1b, the first compound approved for use outside of clinical trials; Interferon-ß-1a; and Copolymer 1.
Experimental agents include other Interferons, Methotrexate, Linomide, MonoClonal AntiBodies, T-Cell Receptor Peptides, and 2-Chlorodeoxyadenosine. Although they have been used effectively to treat exacerbations of MS, CorticoSteroids and Corticotropin are now under evaluation as disease modifying agents.
A second strategy, enhancing ReMyelination by limiting DeMyelination and Oigodendrocyte injury, is represented by Protein Growth Factors.
A third therapeutic approach, improving conduction in DeMyelinated fibers, is represented by the Potassium channel blockers: 4-Aminopyridine and 3,4-DiAminoPyridine.
#7
Clinical Trials Of ImmunoSuppression And ImmunoModulation In MS
Kappos L
J NeuroImmunol 1988 Dec;20(2-3):261-8
Max-Planck-Society, Clinical Research Unit for Multiple Sclerosis,
Wurzburg, FRG
PMID# 3058744; UI# 89067092
Abstract
Although clinical trials in Multiple Sclerosis are extremely difficult to conduct because of the unpredictable course of the disease and the problem of defining and monitoring disease activity, some controlled and several uncontrolled studies suggest that global ImmunoSuppression may result in a small but beneficial effect.
After some methodological considerations the results of recent trials are reviewed especially those concerned with the use of Cyclophosphamide, Azathioprine and Cyclosporine.
Major improvements in our ability to slow-down disease progression may develop through the administration of more sophisticated, phase-adapted therapies using combinations of different ImmunoSuppressive and modulating agents which should be applied in earlier phases of the disease.
Improved monitoring of disease evolution by the addition of Magnetic Resonance Imaging to current clinical scales and by ameliorated Immunological techniques is an indispensable prerequisite of any further therapeutic progress.
#8
The Historical Development Of Interferons As Multiple Sclerosis Therapies
Johnson KP
J Mol Med 1997 Feb;75(2):89-94
Univ of Maryland Hospital,
Baltimore, Maryland 21201, USA
PMID# 9083926; UI# 97237448
Abstract
Human Interferon (IFN) ß is the first therapeutic agent to convincingly reduce the Multiple Sclerosis relapse rate and retard disability.
To achieve this significant treatment advance over 15 years of preliminary work was necessary, encompassing over 15 controlled trials which employed each of the three human Interferons. Important insights into the PathoGenesis of Multiple Sclerosis (MS) were gained, especially from the findings of the single IFN-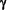 trial. trial.
This short history describes the unfolding of our current understanding of the place for IFNs in the management of MS. The contribution of the patients who have participated is recognized and their courage acknowledged.
The final role for IFN treatment of MS is unclear, but future studies will be required to define the best IFN, optimal dose, and route of administration and patient selection for long-term management of MS.
#9
Non-Specific ImmunoSuppression
And Multiple Sclerosis
Brochet B
Rev Neurol (Paris) 1998 Sep;154(8-9):629-34
Hopital Pellegrin, Service de Neurologie,
CHRU de Bordeaux
PMID# 9809379; UI# 99026907
Abstract
Several series of arguments favor at least partial efficacy of ImmunoSuppression in Multiple Sclerosis. ImmunoSuppression can often treat Experimental AutoImmune Encephalitis, an imperfect model of Multiple Sclerosis.
Certain agents have been shown to affect the pathophysiological processes seen indirectly on Magnetic Resonance Imaging (Mitoxantrone and Campath, for example). Therapeutic trials have their methodological weaknesses but do allow certain conclusions.
The Progressive forms of Multiple Sclerosis are the most widely studied. Massive but short-term ImmunoSuppression does not appear to affect the course of progression but prolonged ImmunoSuppression would appear to slow down the process, at least in responders.
The effect on disease progression is modest and preference should go to well-tolerated treatments. ImmunoSuppression appears to effectively decrease the number of acute episodes and reduce the number of new lesions detectable by Magnetic Resonance Imaging.
The effect of ImmunoSuppression is limited however by the fact that the clinical course of progressive forms depends less on the development of new lesions than on an aggravation of the DeMyelination process and possible Axon Loss within constituted lesions.
This is a further argument favoring early ImmunoSuppressive treatment at a stage when it can be most effective.
#10
Blood Levels Of Soluble ICAM-1
In Multiple Sclerosis
Garcia-Tortosa C, Amrani Y, Casado A, Munoz FJ, Vazquez J
Rev Neurol 1998 Jun;26(154):926-9
Hospital Torrecardenas, Seccion de Neurologia,
Almeria, Espana
PMID# 9658462; UI# 98322598
Abstract
Introduction
Multiple Sclerosis (MS) is a chronic inflammatory disease of the Central Nervous System. The InterCellular Adhesion Molecule plays a fundamental part in the migration of T-Cells to the inflammed tissues.
It is known that there is an increase in the expression of Endothelia Cells and other cells found in lesions of the Central Nervous System of MS patients. ICAM-1 may be detected in its soluble form in Serum and it is of interest to understand its behavior in this liquid since it is easily accessible.
Materials & Methods
We studied the concentration of soluble ICAM-1 in the Serum of patients with MS: one group which had clinical attacks were studied whilst in remission; another had the Chronic/Progressive clinical form and a third group had Optic Neuritis as the probable initial sign of the disorder.
Within the different clinical forms, some patients received treatment with ß-Interferon or Azathioprine and others did not.
Results & Conclusions
We found a significant increase in the levels of soluble ICAM-1 in patients with inactive, untreated MS as compared with normal persons, with no difference found between the different clinical types.
#11
Fatigue & Functional System Involvement In Multiple Sclerosis
Iriarte J, Carreno M, de Castro P
Neurologia 1996 Jun-Jul;11(6):210-5
Rush-Presbyterian-St Luke's Medical Center, Dept of Neurological Sciences,
Chicago, USA
PMID# 8768676; UI# 96344688
Abstract
Fatigue is a frequent complaint in Multiple Sclerosis (MS) patients. Its PathoGenesis is unknown and the question of whether or not there is a specific type of Fatigue in MS is controversial.
The aim of this work was to determine the link between Fatigue in MS and physical and psychological involvement. We studied 50 patients diagnosed of MS category by Poser's Criteria. They were examined using the Kurtzke and Hamilton scales for Depression and anxiety.
We also applied an original scale for assessing the spontaneity, clinical characteristics, severity and frequency of Fatigue. Asthenia, a tendency to feel tired and a worsening of other symptoms were identified as clinical traits.
Using statistical tests for non parametric distribution of data (Spearman's R and Kruskal-Wallis's H coefficients), we found a positive correlation between the characteristics of and severity of Fatigue and Functional System involvement, Anxiety and Depression.
Thirty-one (62%) patients suffered Fatigue, 22 of them spontaneously. Fatigue was the main symptom in 3. and was proportional to Pyramidal involvement (r = 0.41; p < 0.01) and intelligence quotient (r = 0.30; p < 0.03).
Depression and Anxiety were not related to Fatigue (p > 0.05). Patients in the Progressive Phase of disease had higher Fatigue scores than did patients who were stable or in remission. Disease duration was not proportional to Fatigue.
Our results point to a high rate of Fatigue in MS patients. The severity of Fatigue is proportional to Pyramidal involvement and mental decline and is linked to phases of disease progression.
#12
Meta-Analysis Of The Placebo Treated Groups In Clinical Trials Of Progressive Multiple Sclerosis
Weinshenker BG, Issa M, Baskerville J
Neurology 1996 Jun;46(6):1613-9
Mayo Clinic, Dept of Neurology,
Rochester, MN 55905, USA
PMID# 8649559; UI# 96248978
Abstract
The behavior of the control groups can substantially affect the power and outcome of a clinical trial.
We report a meta-analysis of the control groups of four large, double blind, placebo controlled clinical trials of ImmunoSuppressive treatment of Progressive MS to address the sensitivity of five hypothetical definitions of treatment failure (TF).
The rate of TF in the aggregate control groups (n = 427) was 31% when a confirmed increase of 1.0 Expanded Disability Status Scale (EDSS) point was required at the end of the trial; it was 51% when confirmation was not required and TF was allowed at the first point where the criteria for TF were met.
The rate of confirmed TF was 45% when the TF criteria were indexed to baseline EDSS, accounting for the observed differences in staying times at different EDSS levels. We developed models predicting TF in Progressive MS.
In addition to baseline EDSS, the Pyramidal functional score and, for one definition, BrainStem functional score were associated with probability of TF.
#13
EDMUS - A New European Databank
For Multiple Sclerosis
A brief introduction of ongoing and planned multicenter studies within the scope of the "European Concentrated Action for Multiple Sclerosis"
Flachenecker P, Hartung HP
Nervenarzt 1996 Apr;67(4):277-82
Julius-Maximilians-Universitat,
Klinische Forschungsgruppe fur Multiple Sklerose,
Neurologische Klinik und Poliklinik,
Wurzburg
PMID# 8684505, U # 96242668
Abstract
EDMUS, the European Database for Multiple Sclerosis (MS), was established on the occasion of the first European Concerted Action for MS under the auspices of the European Community.
The system is user-friendly and makes it possible to record and retrieve data relevant to MS. Within the framework of another European Concerted Action, several international multicenter trials are currently in progress.
Evalued, a validation of the EDMUS system, assesses the interrater variability; PRIMS examines the influence of pregnancy and the post-partum period on the course of MS; and PRESTIMUS investigates predictive factors in suspected MS.
Further, planned studies include ERAZMUS (early Azathioprine and Interferon-ß treatment in MS) and AZASTOP, which is designed to evaluate the effect of stopping Azathioprine treatment.
#14
Current Therapy Of Multiple Sclerosis
Vass K, Schmied M
Wien Med Wochenschr 1996;146(19-20):514-9
Universitatsklinik fur Neurologie,
Wien
Karl.Vass@AKH-Wien.ac.at
PMID# 9082651; UI# 97142378
Abstract
There is no cure for Multiple Sclerosis (MS). Many therapeutic strategies are known. With exception of high dose CorticoSteroids for the treatment of acute exacerbations most of the concepts are not generally accepted.
Reasons are the variable, often unpredictable natural course of MS, the incomplete understanding of the pathophysiology and the difficult trial design. Yet, new therapeutic agents give hope at least to slow the course of the disease.
#15
Multiple Sclerosis - Status Of Therapy
Reichel D, Kolmel HW
Z Arztl Fortbild (Jena) 1995 May;89(2):151-8
Neurologische Klinik und
Poliklinik am Klinikum Erfurt
PMID# 7610682; UI# 95335054
Abstract
The rational drug-treatment of Multiple Sclerosis on an Immunological basis requires an acquaintance with the different forms of the disease course along with an understanding of the underlying PathoMechanisms as well as a thorough knowledge of the chances and limitations of such a therapy.
Besides some well established treatment regimens other approaches are presented which are currently evaluated in clinical trials and their effects and side effects are discussed.
|