Nerve Fiber Fatigue In Multiple Sclerosis |
|
«¤»¥«¤»§«¤»¥«¤»§«¤»¥«¤»§«¤»¥«¤»§«¤»¥«¤»§«¤»¥«¤» ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯ It is important to be aware that several different causes and types of Fatigue may co-exist, and usually do. Nerve Fiber FatigueNerve Fiber Fatigue in MS is a distinct problem not usually thought of as *Fatigue* and is rarely covered in discussions of MS Fatigue. However, it is a central feature that affects function and significantly contributes to clinical Disability. Nerve Fiber Fatigue has distinct characteristics and a diurnal variation, similar to disease related Fatigue. It usually predates the first clinical MS symptoms, is experienced by most MSers, and worsens as the disease progresses. Nerve Fiber Fatigue is an activity and/or temperature related failure, of DeMyelinated or ReMyelinated Axons to conduct Nerve Impulses (Action Potentials). Even Dr. Charcot, who wrote the classic description of MS in 1868, knew DeMyelinated Axons could conduct. |
|
He stated: "MS very rarely issues in complete blindness. This is especially worthy of notice if you remember. Patches of Sclerosis have been found after death, occupying the whole thickness of Nerve trunks in the Optic Nerves in cases, where during life, an enfeeblement of sight simply had been noted." {1} This apparent discrepancy between symptoms and Lesions, constitutes one of the most powerful arguments that the functional continuity of the Axon is not always permanently interrupted. |
|
Scientific literature regarding Conduction failure in DeMyelinated Axons is old and quite extensive. During an exacerbation, Saltatory Conduction fails along Axons, as they are DeMyelinated; however over time, conduction in most of the surviving Axons is restored. The causes of conduction failure during DeMyelination are incompletely understood but include:
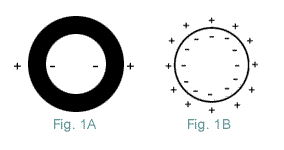
The role if any, of alterations in the ExtraCellular fluid composition, in the inflamed area is unclear. There is evidence the Nodal Axon is damaged by LysoLecithin and other detergent LysoLipids generated, by enzymes present in Inflammation. Various enzymes are released by Inflammatory Cells, including Nitric Oxide, a variety of Proteases, Lipases, NeurAminidase, Phosphatases, and Glycosidases. Of these, Phospholipase appears to produce the most rapid and extensive Myelin damage. Phospholipase also specifically destroys Sodium Channels as measured by SaxiToxin binding {2,3}. Once the acute Inflammatory Response is over, repair processes take over and Nerve conduction resumes. The Axon's InterNodes (Area beneath Myelin) normally contain, very few Sodium channels. Indeed, if Sodium channels on a Myelinated Axon were evenly distributed over its entire length, their density would be much less than half that in most UnMyelinated Axons and would be too few to support conduction {6,7,8}. In order for continuous conduction to develop in a DeMyelinated Axon, the Axon must form additional Sodium channels. This is prerequisite for the restoration of continuous conduction, along a DeMyelinated Axon; but, this alone will not ensure that conduction will occur. Because a DeMyelinated Axon has a giant Capacitance Charge. This increased capacitance results in, a huge increase in the amount of current required, to depolarize its membrane to Threshold. So the current passing down the Axon, from the last Myelinated region is normally insufficient, to discharge a DeMyelinated membrane's capacitance to Threshold. This is more easily understood if you regard the Axolemma and Myelin, as the Di-Electric of a tubular Capacitor. It separates the charged plates (the positive ExtraCellular Fluid from the Axon's negative interior). By definition: Capacitance is inversely proportional to the distance between two plates of a Capacitor (Fig 1). Therefore, the capacitance of a DeMyelinated Axon is many times that of a Myelinated Axon. Whose numerous Myelin layers, electrically insulate the negatively charged Axon's interior, from the positively charged ExtraCellular Fluid.
Current passing along the last Myelinated segment to a DeMyelinated segment is mainly from the last Node. On a large Axon, this distance can be 2 mm away (Fig 2); so the generated current is insufficient to DePolarize the DeMyelinated membrane (Axolemma) to Threshold. Thus, conduction fails at the junction of the Myelinated and DeMyelinated segments; because the number of Sodium channels in the DeMyelinated Axolemma are insufficient, for NonSaltatory Conduction (Fig 2C). In ElectroPhysiological literature, this conduction problem is termed Impedance Mismatch.
Conduction Block is overcome by ReMyelination at the Plaque margins (Figure 2D and 2E) and an increase in the number of Sodium channels in the DeMyelinated Axon {9}. (Also See: Conduction Block) New Myelin (ReMyelination) has very short InterNodes, which allows Summation of the current from several Nodes. This results in an increase of the Sodium current, overcoming the Impedance mismatch and initiates continuous conduction in the DeMyelinated Axon.
When a Nerve Impulse triggers a Node, essentially all Sodium channels sequentially open and Sodium pours in, DePolarizing the Axon. But, the rate Sodium channels close is much more temperature dependent. Such that with an increase in temperature, they close faster. Decreasing the time in which current can flow, which decreases total current producion, and is another cause of conduction failure. Cooling has the opposite effect, increasing the time that channels are open, which improves efficient current production and prolongs continuous conduction. Studies of experimentally DeMyelinated Axons show Temperature Sensitivity, such that a rise in temperature of as little as 0.5°C above normal, will cause conduction failure in some DeMyelinated Axons {11}. It is this Fiber Fatigue that accounts for the problem many MSers have, in walking more than short distances, or performing extended physical activity, and is know to us as heat sensitivity. When Fiber Fatigue occurs and Axons stop conducting, the legs simply will not move, until the Axons are rested enough and begin conducting again {13}. This lack of stamina is more pronounced, during the early stages of recovery from an exacerbation. You may only be able to make a particular movement once or twice initially; but over the next few months, you will be able to increase the number of repetitions and be near your former functioning level. Fiber Fatigue is often physically limiting and is markedly influenced by body core temperature; a cool environment may help, particularly during physical activity. Studies show that individuals can exercise longer with appropriate cooling, most find a cool swim enables better functioning for hours. It lowers the core temperature and causes cooling and VasoConstriction in the extremities, which serve as heat sinks, keeping your body temperature lower for some time. |
|