#2
A Randomized, Controlled Trial Of Oral high-dose MethylPrednisolone In Acute Optic Neuritis
Sellebjerg F, Nielsen HS, Frederiksen JL, Olesen J
Neurology 1999 Apr 22;52(7):1479-84
University of Copenhagen, Department of Neurology, Glostrup Hospital, Denmark
PMID# 10227638; UI# 99242177
Abstract
Objective
To assess the efficacy of oral high-dose MethylPrednisolone in acute Optic Neuritis (ON).
Background
It has been determined that Oral high-dose MethylPrednisolone is efficacious in attacks of MS.
Methods
A total of 60 patients with symptoms and signs of ON with a duration of less than 4 weeks and a Visual Acuity of 0.7 or less were randomized to treatment with placebo (n = 30) or Oral MethylPrednisolone (n = 30; 500 mg daily for 5 days, with a 10-day tapering period).
Visual function was measured and symptoms were scored on a Visual Analog Scale (VAS) before treatment and after 1, 3, and 8 weeks.
Primary efficacy measures were Spatial Vision and VAS scores the first 3 weeks (analysis of variance with baseline values as the covariate), and changes in Spatial Vision and VAS scores after 8 weeks. A significance level of p < 0.0125 was employed.
Results
The VAS score (p = 0.008) but not the Spatial Visual Function (p = 0.03) differed in MethylPrednisolone- and placebo-treated patients during the first 3 weeks.
After 8 weeks the improvement in VAS scores (p = 0.8) and Spatial Visual function (p = 0.5) was comparable with MethylPrednisolone- and placebo-treated patients.
A post hoc subgroup analysis suggested that patients with more severe baseline Visual deficit and patients treated early after onset of symptoms had a more pronounced response to treatment.
The risk of a new DeMyelinating attack within 1 year was unaffected by treatment. No serious adverse events were seen.
Conclusion
Oral high-dose MethylPrednisolone treatment improves recovery from ON at 1 and 3 weeks, but no effect could be demonstrated at 8 weeks or on subsequent attack frequency.
#3
Effect Of MethylPrednisolone On Multiple Sclerosis
Lujan S, Masjuan J, Roldan E, Villar LM, Gonzalez-Porque P, Alvarez-Cermeno JC
Mult Scler 1998 Jun;4(3):239-42
Ramon y Cajal Hospital, Dept of Immunology, Madrid, Spain
UI# 98435429
Abstract
We studied the effect of IntraVenous MethylPrednisolone (MP) on the expression of the Integrins, LFA-1 and VLA-4, on activated blood T-Lymphocytes in 17 patients with relapses of Clinically Definite Relapsing/Remitting MS (CD/MS).
MP treatment did not induce changes in the expression of CD3+, CD4+, DR, LFA-1 or VLA-4 markers when measured in the total population of Lymphocytes in MS patients in relation to treatment.
Treatment influenced neither the LFA-1 nor VLA-4+ Cells within the CD3+ population.
MP treatment clearly decreased the DR+ CD3+ cells (P < 0.01) and the percentage of DR+ CD3+ Lymphocytes bearing VLA-4+ (P < 0.01).
However, this was not the case when we studied the percentage of Lymphocytes which expressed LFA-1.
GlucoCorticoids did not influence the mean intensity of the expression of the two Integrins quantified in either total or DR+ CD3+ Lymphocytes.
Although, further research seems warranted to investigate a possible effect of MP on Lymphocyte Integrin Function.
This work corroborates the idea that MP treatment may interfere with the mechanisms of T-Cell migration into CNS, thus modulating the activity of Multiple Sclerosis.
#4
Sellebjerg F, Frederiksen JL, Nielsen PM, Olesen J
Neurology 1998 Aug;51(2):529-34
University of Copenhagen, Department of Neurology, Glostrup Hospital, Glostrup Copenhagen, Denmark
PMID# 9710030; UI# 98373804
Abstract
Objective
There is only limited evidence from adequately controlled clinical trials to support high-dose MethylPrednisolone therapy for attacks of Multiple Sclerosis (MS) and none supporting Oral administration.
We assessed the effect of Oral high-dose MethylPrednisolone therapy in attacks of MS.
Methods
Twenty-five patients with an attack of MS lasting less than 4 weeks were randomized to placebo treatment.
Twenty-six patients received Oral MethylPrednisolone (500 mg once a day for 5 days with a 10-day tapering period).
The patients received scores on the Scripps Neurological Rating Scale (NRS) and Kurtzke Expanded Disability Status Scale.
The symptoms were scored on a Visual Analog Scale (VAS) before treatment and after 1, 3, and 8 weeks of treatment.
Primary efficacy measures were NRS and VAS scores in the first 3 weeks and changes in NRS score and answers to an efficacy questionnaire administered after 8 weeks of treatment.
Results
Changes in NRS scores among MethylPrednisolone- and placebo-treated patients differed significantly in the first 3 weeks and after 8 weeks (p = 0.005 and p = 0.0007).
VAS scores the first 3 weeks and treatment efficacy after 8 weeks also favored a beneficial effect of MethylPrednisolone treatment (p = 0.02 and p = 0.05).
After 1, 3, and 8 weeks, 4%, 24%, and 32% in the placebo group and 31%, 54%, and 65% in the MethylPrednisolone group had improved one point on the Expanded Disability Status Scale score (all p < 0.05). No serious adverse events were seen.
Conclusion
Oral high-dose MethylPrednisolone is recommended for managing attacks of MS.
#5
Serial Analysis Of Cytokine mRNA Profiles In Early Multiple Sclerosis
Kahl KG, Kruse N, Toyka KV, Rieckmann P
J Neurol Sci 2002 Aug 15;200(1-2):53-5
Julius-Maximilians University, Clinical Research Unit for Multiple Sclerosis and NeuroImmunology, Department of Neurology, Wurzburg, Germany
PMID# 12127676; UI# 22125972
Abstract
In this pilot study, we serially determined the Cytokine messenger RNA (mRNA) expression pattern in whole blood samples from 12 patients with Clinical Isolated Syndrome suggestive of early Multiple Sclerosis (MS) using a new sensitive quantitative Polymerase Chain Reaction (PCR) method.
Significantly higher levels of Tumor Necrosis Factor-alpha (TNF- ; x5.1), Interferon-gamma (IFN- ; x5.1), Interferon-gamma (IFN-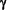 ; x4.8) and InterLeukin-10 (IL-10; x5.6) mRNA were detected in MS patients at the time of a relapse compared to healthy controls. ; x4.8) and InterLeukin-10 (IL-10; x5.6) mRNA were detected in MS patients at the time of a relapse compared to healthy controls.
Treatment with i.v. MethylPrednisolone (MP) led to an increase of IL-4 mRNA and a significant decrease of IFN-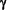 and TNF- and TNF- mRNA expression. mRNA expression.
In this cohort of clinically stable patients, ProInflammatory Cytokines remained low during the 1-year follow-up period.
As several indications point to a Cytokine dysregulation in MS, quantitative analysis of Cytokine mRNA profiles in whole blood samples by real time PCR may be a useful Immunological marker to monitor disease activity in future therapeutic trials in MS.
#6
A Prospective Randomized Trial Of Megadose MethylPrednisolone And High Dose Dexamethasone For Traumatic Optic Neuropathy
Chuenkongkaew W, Chirapapaisan N
J Med Assoc Thai 2002 May;85(5):597-603
Mahidol University, Faculty of Medicine Siriraj Hospital, Department of Ophthalmology, Bangkok, Thailand
PMID# 12188391
Abstract
Purpose
To determine whether the improvement in Visual Acuity obtained when using high dose Dexamethasone in the treatment of traumatic Optic Neuropathy was comparable to that of megadose MethylPrednisolone.
Method
A total of forty-four patients with traumatic Optic Neuropathy were prospectively randomized and selected to receive intravenous high dose Dexamethasone or megadose MethylPrednisolone within 2 weeks of injury.
Age, gender, cause of injury, interval from injury to treatment, initial, post-pulse, and final Visual Acuity were analysed statistically to compare the Dexamethasone and MethylPrednisolone groups.
Results
The mean interval to treatment was not significantly different (p=0.28) for the Dexamethasone group at 5.5 days compared to the MethylPrednisolone group at 4.1 days.
Visual improvement of at least two lines of the Snellen chart or two levels of unmeasured Visual Acuity was shown in 9 patients (37.5%) of the Dexamethasone group and 10 patients (50%) of the MethylPrednisolone group.
There was no statistically significant difference between the initial and post-pulse Visual Acuity (p=1.0) and the initial and final visual outcome (p=0.60) in the Dexamethasone group compared with the MethylPrednisolone group.
Conclusion
There was no significant difference in the Visual Acuity obtained after treatment with intravenous Dexamethasone or MethylPrednisolone for traumatic Optic Neuropathy.
#7
Low-dose IntraVenous MethylPrednisolone Or Conservative Treatment In The Management Of Traumatic Optic Neuropathy
Yip CC, Chng NW, Au Eong KG, Heng WJ, Lim TH, Lim WK
Eur J Ophthalmol 2002 Jul-Aug;12(4):309-14
The Eye Institute, National Healthcare Group, Tan Tock Seng Hospital, Singapore, Singapore
PMID# 12220002
Abstract
Purpose
To compare the efficacy of low-dose IntraVenous MethylPrednisolone or conservative treatment in the management of traumatic Optic Neuropathy.
Methods
A non-randomized retrospective study of 21 patients (21 eyes) with traumatic Optic Neuropathy treated between October 95 and November 97 in a Tertiary Ophthalmology Unit.
Traumatic Optic Neuropathy was defined as Traumatic Visual Loss with Afferent Pupillary Defect in the absence of direct injury to the Globe or Optic Nerve.
The median follow-up period was one year. Nine patients were treated with 125-250 mg MethylPrednisolone 6-hourly intravenously for a mean of 3.3 days (range 2-5 days) and 12 patients were treated conservatively.
Visual Acuity (VA) was measured with a Snellen chart before and after treatment at each follow-up visit. Visual recovery was defined as an improvement of 2 or more Snellen lines one week post-injury or later.
Results
The patients' mean age was 37.1 years (range 12-65 years). There were more males (90.5%) than females (9.5%).
Traumatic Optic Neuropathy was in 12 right eyes and 9 left eyes. The cause of injury included traffic accidents (52.4%), falls (28.6%), assault (14.2%) and others (4.8%).
The mean interval between the injury and Steroid therapy was 3.6 days (range 1-11 days). Visual recovery was observed in 44.4% of eyes treated with MethylPrednisolone and in 33.3% treated conservatively (p = 0.673, Fisher's exact test).
Conclusions
Intravenous MethylPrednisolone at the dosage and duration used in this retrospective study did not significantly improve the Visual recovery of Eyes with traumatic Optic Neuropathy compared to conservative treatment.
However, this small sample may not be sensitive enough to detect a small difference in Visual recovery rates, and further studies with larger samples may be warranted.
#8
CD95/Fas Expression On Peripheral Blood T-Lymphocytes In Multiple Sclerosis: Effect Of High-Dose MethylPrednisolone Therapy
Petelin Z, Brinar V, Petravic D, Zurak N, Dubravcic K, Batinic D
Clin Neurol NeuroSurg 2004 Jun;106(3):259-62
University Department of Neurology, Zagreb University Hospital Center, Kispaticeva 12, 10 000 Zagreb, Croatia
PMID# 15177780
Abstract
Recent data indicate that the Apoptotic process, mediated by the CD95/Fas Cell Surface Receptor, is impaired in activated Lymphocytes of patients with Relapsing/Remitting Multiple Sclerosis.
Using Flow Cytometric-ImmunoPhenotyping, we analyzed the expression of CD95/Fas on peripheral blood CD4+ and CD8+ T-Lymphocytes (PBL) in 10 MS patients in relapse, and the effect of pulse CorticoSteroid therapy on the Apoptosis of AutoReactive Lymphocytes.
The proportions of CD8+ and CD8+CD95+ T-Lymphocytes were significantly higher in MS patients in relapse before than after pulse CorticoSteroid therapy.
Conversely, the proportions of CD4+ and CD4+CD95+ T-Cells were significantly lower before than after therapy, but not significantly different from healthy persons.
The different expression of CD95/Fas on peripheral blood CD8+ T-Lymphocytes in RRMS and in healthy controls suggests a possible involvement of Apoptosis in the pathogenesis of MS.
Our results also show that pulse CorticoSteroid therapy influences the CD95/Fas expression on CD8+ and CD4+ T-Lymphocytes in patients with RRMS.
#9
Cerebral Volume Changes In Multiple Sclerosis Patients Treated With High-Dose IntraVenous MethylPrednisolone
Hoogervorst EL, Polman CH, Barkhof F
Mult Scler 2002 Oct;8(5):415-9
VU Medical Center, Department of Neurology, Amsterdam, The Netherlands
PMID# 12356209
Abstract
Objective
Multiple Sclerosis (MS) patients develop varying degrees of Cerebral Atrophy, which may already begin at disease onset.
The purpose of this study is to examine the effect of Steroid treatment on Cerebral volume in MS patients.
Methods
Thirty-five MS patients participating in a clinical trial of oral Interferon-beta, which induded monthly MRI, were included in this study.
They suffered from an acute relapse and were treated with IntraVenous MethylPrednisolone (IV-MP); 13 of the patients were treated with oral Prednisolone tapering after IV-MP.
The last MRI scan before and the first (and second for oral tapering patients) scan after IV-MP treatment were used for measuring Parenchymal Fraction (PF) and Ventricular Fraction (VF). Changes in PF and VF were analysed using Student's t test.
Results
For the total population no significant changes in PF or VF were found.
However, the subgroup of patients receiving oral tapering after IV-MP showed changes, compatible with Atrophy in both PF and VF, that were significant immediately after IV-MP treatment.
And still persisted (though not statistically significant anymore) after a mean interval of 30 days.
The magnitude of these changes was about the same as the annual change in Cerebral volume as reported in natural history studies.
Conclusion
Our data indicate that short courses of intravenous Steroids (restricted to three or five days) have no major impact, whereas prolonged treatment with oral tapering does significantly affect Brain Volume.
These findings are important for longitudinal studies and clinical trials in which Brain volume is used as an outcome measure.
#10
The Bioavailability Of IV MethylPrednisolone And Oral Prednisone In Multiple Sclerosis
Morrow SA, Stoian CA, Dmitrovic J, Chan SC, Metz LM
Neurology 2004 Sep 28;63(6):1079-80
University of Western Ontario, Department of Clinical Neurosciences, London, Ontario, Canada
PMID# 15452302
Abstract
Oral Prednisone (1)might be a convenient, inexpensive alternative to IV MethylPrednisolone (IVMP) if the bioequivalent dose was known.
We compared the total amount of Steroid absorbed after 1250 mg oral Prednisone vs 1 gram IVMP in 16 patients with Multiple Sclerosis (MS).
At 24 hours, the mean area under the concentration-time curve (AUC), the main component of bioavailability, did not differ between groups (p = 0.122). This suggests that the amount of absorbed CorticoSteroid is similar after either Steroid at these doses.
#11
Clinical Impact Of IntraVenous MethylPrednisolone In Attacks Of Multiple Sclerosis
Nos C, Sastre-Garriga J, Borras C, Rio J, Tintore M, Montalban X
Mult Scler 2004 Aug;10(4):413-6
Unitat de Neuroimmunologia Clinica, Hospital Vall d'Hebron, Barcelona, Spain
PMID# 15327039
Abstract
Background
IntraVenous MethylPrednisolone (IVMP) has been shown to hasten recovery from attacks of Multiple Sclerosis (MS) without altering the long term evolution of the condition.
However, there is little evidence available to suggest which patients are more likely to benefit from IVMP treatment.
Objective
To measure clinical change after IVMP treatment and to identify predictors of good outcome.
Methods
Retrospective open-label study of medical records from 51 patients with clinically isolated syndromes or Relapsing/Remitting MS treated with IVMP for an acute attack (54 attacks).
Results
A measurable neurological improvement was observed at one month in 44% of these attacks; the only predictor of Expanded Disability Status Scale (EDSS) change at one month was the severity of the attack.
Conclusion
Attack severity predicts good response to IVMP when measured by means of EDSS.
#12
Change Of InterLeukin-4 And InterLeukin-12 Levels After Therapy Of Multiple Sclerosis Relapse With MethylPrednisolone
Bartosik-Psujek H, Magrys A, Montewka-Koziol M, Stelmasiak Z
Neurol Neurochir Pol 2005 May-Jun;39(3):207-12
Katedra i Klinika Neurologii, Akademia Medyczna w Lublinie
PMID# 15981158
Abstract
Background And Purpose
In the pathomechanism of Multiple Sclerosis (MS), a vital role is attributed to AutoImmune responses.
Higher activity of the disease is connected with an increased activity of proinflammatory Cytokines while in remissions anti-inflammatory Cytokines dominate.
The aim of the study was to evaluate the effects of MethylPrednisolone treatment on the level of IL-4 and IL-12 and to determine whether their levels are related to the degree of disability and may be prognostic factors in the assessment of relapse sequelae.
Material And Methods
The study included 32 patients with MS (according to McDonald's criteria) with relapses and remissions (17 patients with relapse who received MethylPrednisolone in the dose of 1.0 g i. v. on 5 consecutive days and 15 patients in remission).
The control group consisted of 15 patients with non-inflammatory diseases of the Nervous System. The levels of Cytokines were determined by ELISA method using the Pharmingen kits.
The examinations of patients in relapse were conducted before and after Steroid therapy; the remaining patients were examined only once.
Results
In the relapse a visible increase in serum IL-12 level (p < 0.05) was observed; its level after MethylPrednisolone treatment was found to be significantly decreased (p < 0.001).
The level of IL-4 was not significantly affected by Steroid therapy. There was no relation between the severity of disability and Cytokine levels.
Conclusions
The level of IL-12 increases in MS relapses and decreases after MethylPrednisolone therapy.
The changes in IL-4 and IL-12 levels are the manifestations of inflammatory reactions connected with the relapse; however, they do not directly indicate the extent of damage to CNS.
They cannot be treated as a prognostic factor allowing to anticipate the consequences of the relapse.
|